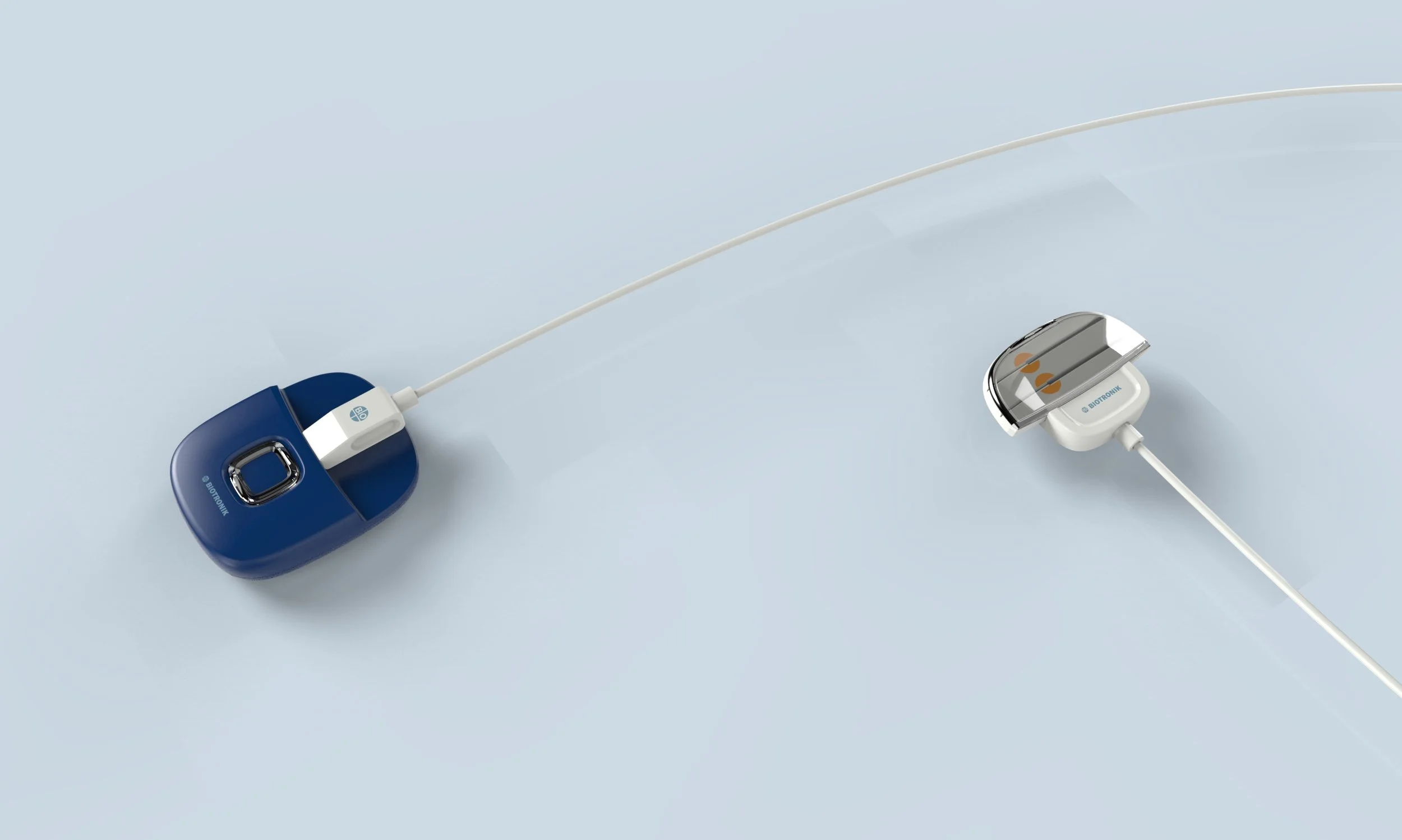
Biotronik Spinal Cord Stimulation System
MSEII/Biotronik is currently in development of a Spinal Cord Stimulation System that requires an External Pulse Generator (EPG) for patients to use during their trial period to determine their eligibility for an implantable pulse generator (IPG). Should the patient be approved for the IPG it will require periodic charging, transcutaneously, by use of a Charging device (Charger) and belt type device (Belt) to locate the Charger in proximity to the IPG for optimum charging. The Charger will also require periodic charging of its internal battery and Karten Design (K:D) envisions this being accomplished by way of a docking station.
Our team reviewed in-depth design research, interviewed opinion leaders, surgeons, technicians, and patients to understand the current spinal cord stimulation (SCS) treatment for chronic back pain. Armed with insights that revealed opportunities to improve on the efficacy, acceptance, and usability of SCS treatment, we began defining Biotronik’s new system and the user experience around it.
As the design lead, I was tasked with creating brand identity for suite of products and preliminary mechanical engineering of the EPG, Charger, Charging Dock, Trial Stimulator and Lead Adapter.
Key activities included: Visual Form Language, Branding, Co-Development, Human Factors, Voice Of The Consumer Research, Construction Strategy, Modularity, Optimized Design For Manufacture And Serviceability.
SCS Charger and Dock
EPG Concept
This configuration allows lead placement and testing during procedure.
Connection to EPG